 Many safety-net health care organizations rely on savings generated from participation in the 340B Drug Pricing Program (340B program) to stretch scarce federal funding and resources, reach more eligible patients, and provide more comprehensive services to their communities.
Many safety-net health care organizations rely on savings generated from participation in the 340B Drug Pricing Program (340B program) to stretch scarce federal funding and resources, reach more eligible patients, and provide more comprehensive services to their communities.
While the 340B program comes with many benefits, it also has significant regulatory and compliance requirements. Failure to meet these requirements can result in repayment to manufacturers and possible termination from the 340B program.
Background
Congress expanded the Medicaid drug rebate program to safety-net providers in 1992 to provide relief from high drug costs afforded through Medicaid rebate regulations. These regulations were an enactment of Section 340B of the Public Health Service Act, created under Section 602 of the Veterans Health Care Act of 1992.
Manufacturers enter into pharmaceutical pricing agreements (PPA) with the US Secretary of Health and Human Services. Rather than a rebate, manufacturers agree to provide a front-end discount on covered outpatient drugs purchased by participating health care providers, known as covered entities (CE), under the 340B program.
The Health Resources Services Administration (HRSA) is responsible for 340B program oversight, and the Office of Pharmacy Affairs (OPA) operates as the administrator of the 340B Program under HRSA. The Centers for Medicare and Medicaid (CMS) has authority over the billing requirements of 340B program-covered drugs.
Participation in the 340B Program
Not-for-profit health care organizations that have specified federal designations or receive funding from specified federal programs are eligible to participate in the 340B program.
A qualifying safety-net provider can apply to participate in the 340B program as a CE through the HRSA–OPA website. Qualifying entities include, but aren’t limited to, Federally Qualified Health Centers, Critical Access Hospitals, Title X Family Planning Clinics, and Rural Referral Centers.
Once a CE is approved to participate in the program, it must meet myriad compliance requirements to maintain eligibility. These include the following:
- Performing an annual recertification
- Maintaining up-to-date and accurate records in the in the Office of Pharmacy Affairs Information System (OPAIS)
- Preventing diversion to ineligible patients
- Prohibiting duplicate discounts
- Being prepared for a program audit
Certain CEs—disproportionate share hospitals, freestanding cancer hospitals, and children’s hospitals—are prohibited from participation in a group purchasing organization for covered outpatient drugs. Further, a CE that enters into an agreement with a contract pharmacy to dispense outpatient pharmaceuticals to patients of the CE is responsible for the contract pharmacy’s compliance with 340B program requirements.
Compliance and Audit Focus Areas
Because compliance with the 340B program is complex, it’s important for CEs to understand the program’s primary compliance focus areas to help prepare for a HRSA audit.
There are seven elements of an effective compliance program that CEs can review to help navigate 340B program requirements. These elements are outlined by the Office of Inspector General, US Department of Health and Human Services’ Health Care Fraud Prevention and Enforcement Action Team. By paying attention to these areas, CEs can begin to develop a strong compliance program. Here’s an overview of each element when applied to the 340B program.
Seven Elements of Compliance
- Review, update, and communicate policies and procedures. Document, maintain, and communicate policies and procedures for your 340B program to the applicable workforce. Periodically review and update policies and procedures. Annual reviews and updates are highly recommended.
- Update your information in the OPAIS database. Routinely review and update the OPAIS database to help ensure outpatient facility and contract pharmacy information is up to date and accurate, including correct spelling and accurate address information. It’s important to update any new or closed sites.
- Recertify your information. Recertify information in the OPAIS database annually and within the designated timeframes.
- Audit and monitor program-covered drugs. Develop a routine auditing and monitoring process to validate that 340B program-covered drugs weren’t resold or otherwise transferred to an ineligible patient. This includes activities by contract pharmacies because CEs are liable for the actions of contract pharmacies. Reviews should be performed quarterly and at least once each year.
- Audit and monitor accounts. Routinely audit and monitor to identify potential duplicate discounts within your organization and your contract pharmacies. Be aware of your state’s Medicaid reporting requirements for identifying 340B program-covered drugs.
- Document activities. Document all self-auditing and monitoring activities and make sure documentation is readily available. Retain auditable records of overall compliance with all elements of the 340B program.
- Monitor contract pharmacies. Monitor contract pharmacies internally and contract for an external audit to determine if contract pharmacies are putting your program at risk.
These seven compliance areas can help CEs build out and demonstrate compliance with all requirements of the 340B program. In addition, Apexus, HRSA’s 340B Prime Vendor, offers many resources to help CEs with program compliance.
HRSA Audits and Program Findings
HRSA–OPA contracts with auditors to perform 340B program audit fieldwork through desk and on-site audits. The below graph reflects the total number of program audits conducted in each of the past five years, based on program integrity information on the HRSA website.
HRSA Audits Completed Each Year
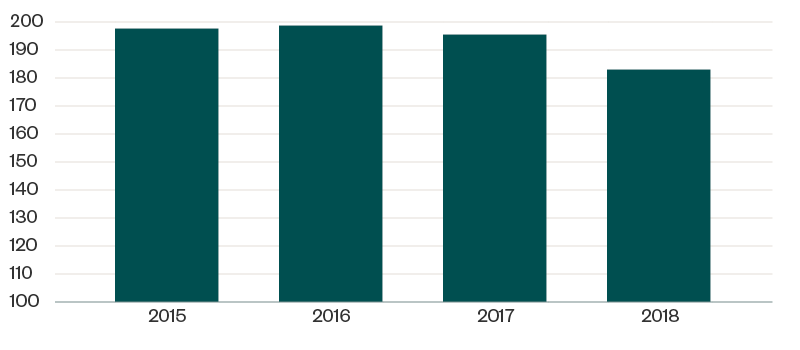
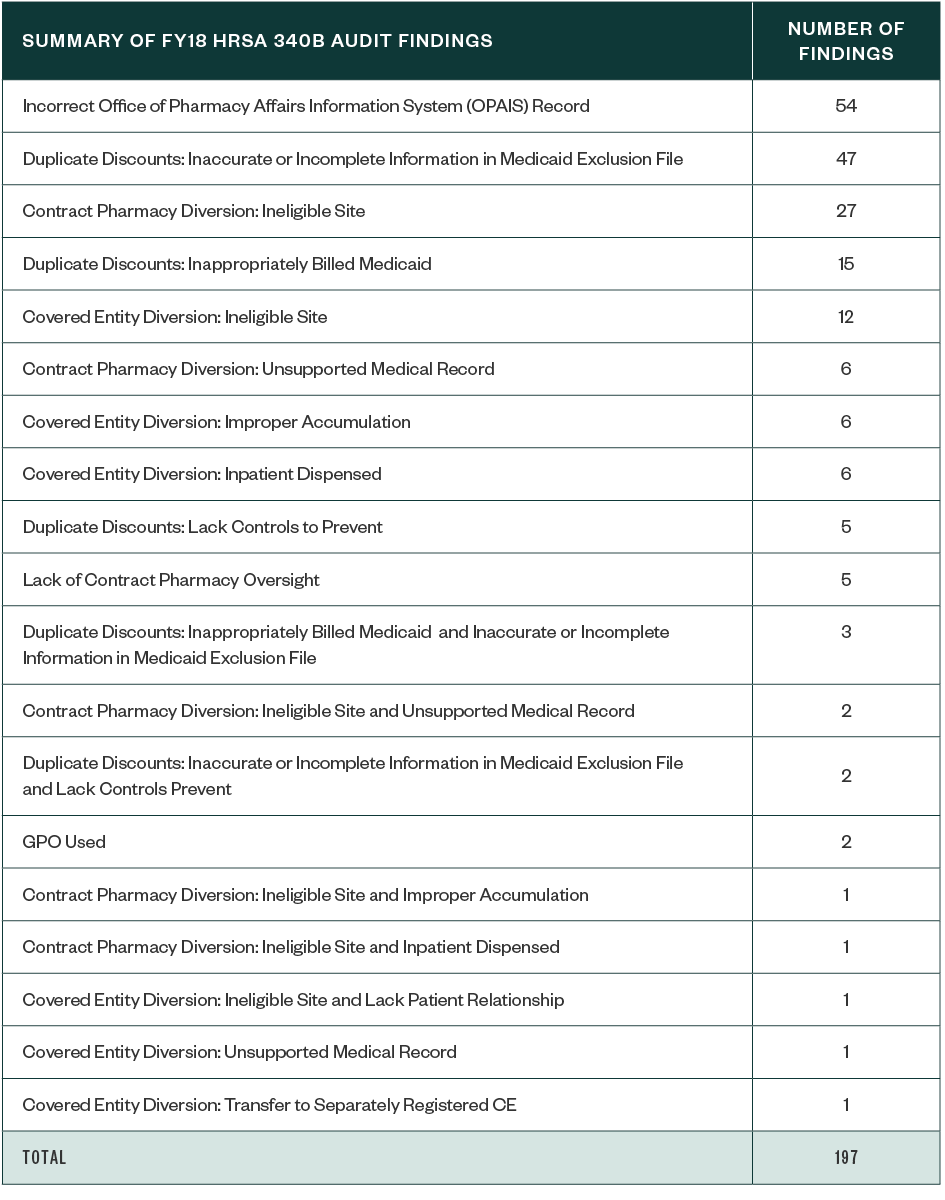
Of the 183 CEs audited in 2018, a total of 197 identified program findings were attributed to 124 CEs. This means many CEs had multiple areas of identified noncompliance, as reflected in the below table.
Of the HRSA’s 197 program findings, sanctions included required repayment to manufacturers as well as termination of contract pharmacies and ineligible offsite outpatient facilities from the 340B program. The below table reflects the number of instances of each type of sanction. A CE could also receive more than one sanction, depending on the findings.

In our experience consulting with CEs, and as indicated on HRSA–OPA, it isn’t possible to predict when a CE will be subject to a HRSA-OPA 340B program audit. That’s why implementing a strong compliance program and regularly auditing and monitoring the 340B program are the most effective ways a CE can prepare for a HRSA audit. A CE’s documentation should also be in order and readily available, including up-to-date policies and procedures as well as evidence of auditing and monitoring activities.
Next Steps
Health care organizations can benefit from investing in resources to identify how the regulations apply to their individual operations. This includes analyzing the internal controls surrounding the program to prevent and mitigate noncompliance.
Some of the best frameworks organizations can use to analyze, refine, and document their internal controls include the following:
- HRSA–OPA
- Apexus
- Internal Control–Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO)
- The US Government Accountability Office’s Standards for Internal Control in the Federal Government (Green Book)
We’re Here to Help
For more detail on 340B program, including compliance, eligibility, diversion, duplicate discounts, and policies and procedures, contact your Moss Adams professional.