
With the global economic upheaval and new industry challenges, it’s imperative that life sciences companies stay ahead of ongoing developments and compliance dynamics to remain competitive. For those looking to go public or make recurring filings, maintaining sound adherence to SEC standards is key.
These changes, coupled with intense industry competition, mean life sciences companies should understand the nature of SEC scrutiny made on past filings to reduce the possibility of delays and additional cost of compliance arising from SEC comments on their own initial public offering (IPO).
In our report, Under the Microscope: An Analysis of SEC Comment Letter Trends Among Middle-Market and Pre-IPO Life Sciences Companies, we look at SEC comments directed toward Forms S-1, 10-K, 10-Q, and 20-F filings made by life sciences companies during a review period from May 1, 2019, to April 30, 2020. Comments were analyzed by frequency as a way to identify the most prominent topics under SEC scrutiny.
Below, we cover key findings in the 2019–2020 SEC report, comparisons to findings from prior years, and implications for middle-market and pre-IPO life sciences companies.
Initial Findings
Throughout 2019–2020 report, R&D made up the largest area of SEC focus, with a strong emphasis on companies’ clinical trials. Comments related to process compliance and licensing agreements also saw a relative increase in focus, while those directed toward entity background and risk disclosures dipped slightly.
Comment Categories
SEC comments toward Forms S-1, 10-K, 10-Q, and 20-F filings for 2019–2020 stood at 799. These were largely spread across several key categories, of which comments related to R&D were the most prominent, garnering a 19.1% share. Much of the focus here derived from understanding companies’ results from ongoing clinical trials and studies, along with their developmental pipelines and upcoming products.
As in the 2018–2019 study, process compliance was the next major category with majority comments, at a share of 17.8%. These comments required filers to make requisite disclosures throughout their prospectuses, including filing all material information. This category was followed by comments requiring disclosure on entity background, management’s discussion and analysis (MD&A), the actual offering, and any current or anticipated risks related to the business.
Information around licensing agreements, shareholders’ equity, underlying patents, and revenue recognition collectively constituted another significant chunk of SEC scrutiny, followed by various other comments targeting firm-specific controls and regulatory features.
The following infographic depicts a breakdown of the total 799 comments analyzed, according to category and frequency.
Overview of SEC Comment Categories
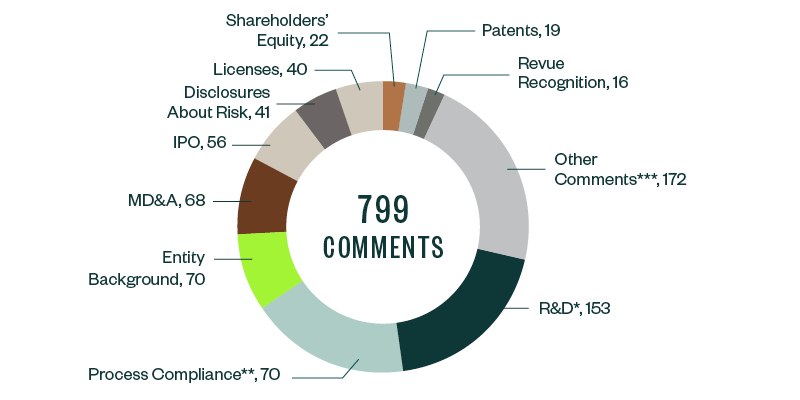
*R&D comments relate to clinical trials and studies, FDA filings and communication, product pipeline, products and services, and other highly firm-specific information.
**Comments related to process compliance tend to be more administrative and formulaic, but, because of the sheer volume of these comments, companies have an opportunity to significantly reduce filing delays by understanding the nature of scrutiny under this topic and taking the appropriate steps to comply.
***Other recurring comments include those related to emerging growth companies, controls and procedures, proxy disclosures, and material contracts.
Significant Shifts
A number of topics saw a slight-to-significant shift in focus when compared to 2018–2019 SEC comments, with the positive or negative variance measured as a ratio to the total number of comments. These categories include R&D, entity-related information, licensing agreements, risk-based disclosures, and process compliance.
Significant Shifts in SEC Focus for Overall Filings | By Ratio of Comments
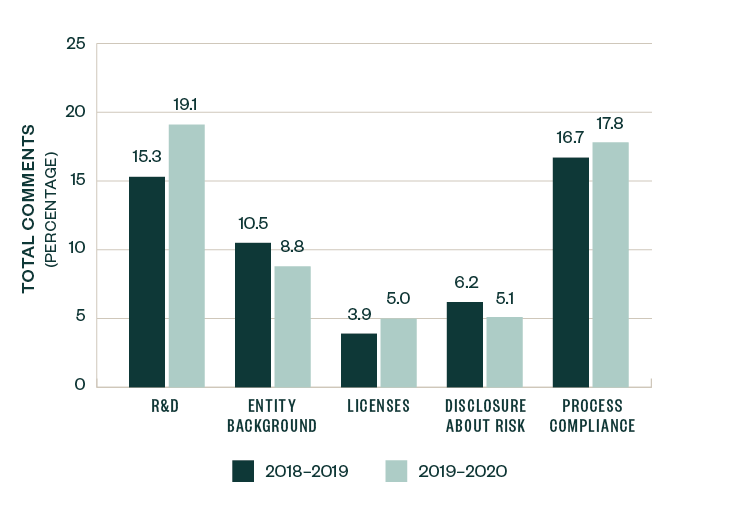
Comments related to R&D saw an increase in focus by 3.8%, while those related to licenses and process compliance increased by 1.1%. Comments directed toward entity background and risk disclosure slightly declined by 1.7% and 1.1%, respectively.
These shifts, however, were relatively marginal in size, highlighting that the nature and composition of comments over the last two years remained fairly consistent.
Filing Type
Similar to the 2018–2019 report, S-1 filings continued to lead in relation to SEC scrutiny. Of the total 799 comments analyzed in the study, 86%—or 684 comments—were directed at S-1 filings, slightly down from a share of 91% in 2018–2019. The remaining 14% of comments were directed toward Forms 10-K, 10-Q, and 20-F filings.
Percentage of Comments | By Filing Type
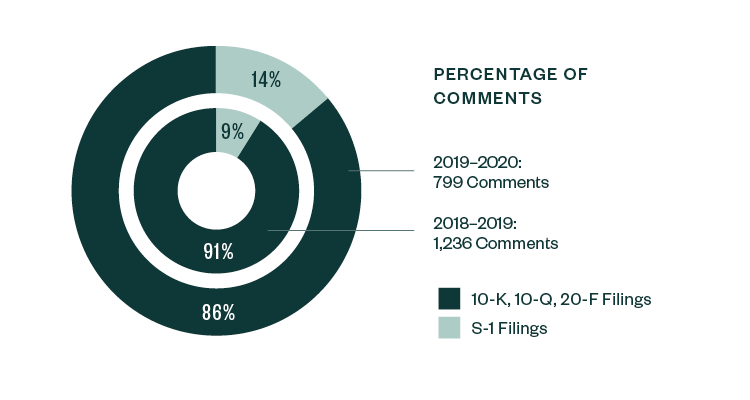
In terms of categorization, the nature of comments continued to vary among pre- and post-IPO companies. Areas like R&D, process compliance, entity background, risk disclosure, and details pertaining to the actual IPO remained very much dominant for S-1 registrants.
Contrastingly, comments under Forms 10-K, 10-Q, and 20-F were more performance-based and operational in nature, requiring companies to:
- Undertake a detailed MD&A
- Explain revenue recognition terms across contracts
- File the necessary certifications
- Carry out requisite compliance checks across their filings
Market Capitalization Range
Breakdown of 10-K, 10-Q, and 20-F Comments | By Market Capitalization Range
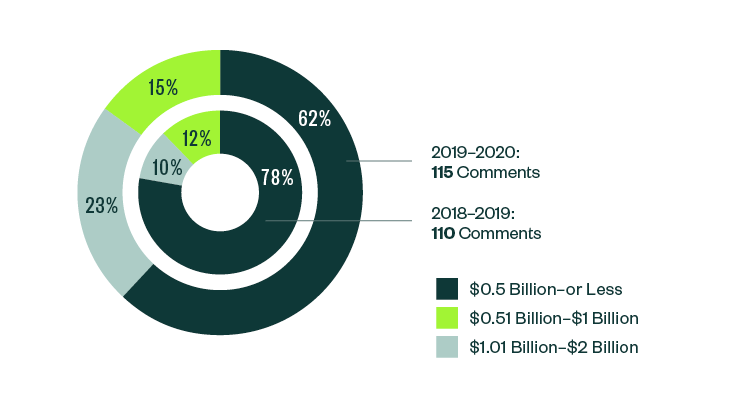
In terms of size, over 62% of all post-IPO comments were centered on companies with a market capitalization of less than $500 million. Of the remaining, 23% were directed toward those with market capitalization between $500 million–$1 billion, while 15% pertained to those with market capitalization that was greater than $1 billion but less than $2 billion.
Even with a slight shift in focus from 2018–2019, smaller companies continued to attract the greatest scrutiny in the 2019–2020 report.
Results by Subindustry
Percentage of Comments | By Subindustry
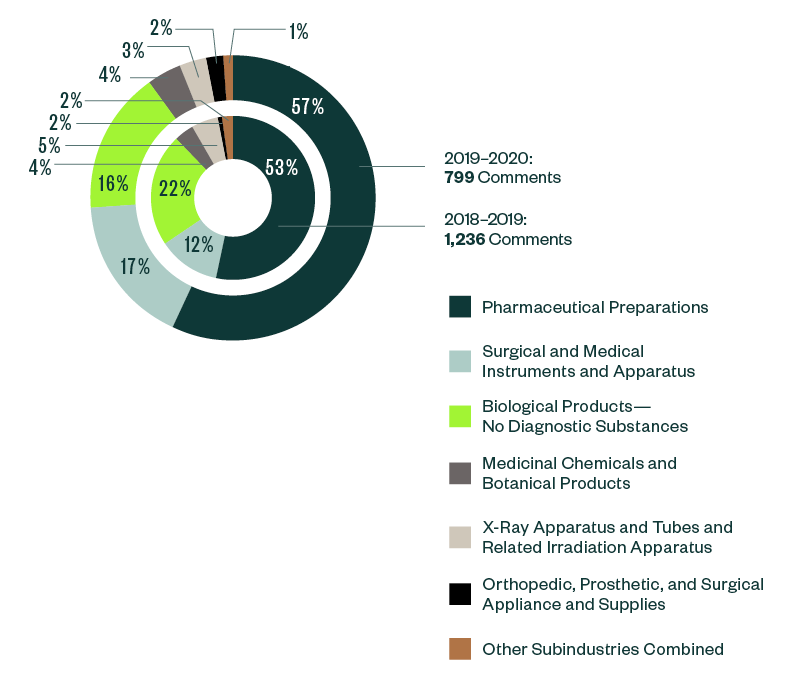
Of all the subindustries analyzed in this study, pharmaceutical preparations continued to grab much of the SEC’s focus. Its share of total comments further increased from 53% in 2018–2019 to 57% in 2019–2020.
Surgical and medical instruments and apparatus stood as the next most significant subindustry, with a share of 17% of the SEC’s comments. This was closely followed by biological products at 16%. There has been an interesting shift of dynamics within these two subindustries, with the ratio of comments in surgical and medical instruments and apparatus increasing by roughly 5% from the 2018–2019 report while the ratio of comments in biological products went down by more than 4%.
The breakdown of other subindustries was relatively small, swinging below 5% of comments.
Additional Content
The SEC considers both the macro environment and business-specific value chains when directing the nature of review. While topics like process compliance were generically commonplace for the entire life sciences sector, others varied among subindustries.
For example, scrutiny in R&D remained more significant for pharmaceutical preparations and biological products, while entity-related disclosures were more in focus for x-ray apparatus and tubes and related irradiation apparatus.
This doesn’t mean, however, that the nature of comments within a subindustry remains static. Certain topics may attract greater scrutiny one year and less the year after. This depends on market dynamics at the time of the SEC’s analysis, which may bring certain issues to the forefront, and the efforts companies undertake to properly address those areas in their statements.
We’re Here to Help
For more information on SEC comments, read our full guide, which covers these topics in greater detail, or contact your Moss Adams professional.